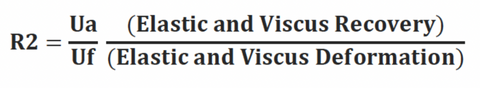Elasticity and Viscoelasticity Cutometer Clinical Study
1.0 OBJECTIVE
The purpose of this study was to evaluate the efficacy of an age-defying serum when used twice-daily and tested over a 28-day period under ambient conditions. Effectiveness of the test product was evaluated objectively on a group of 35 subjects. Elastic and viscoelastic properties of the skin were measured as a function of skin flexibility and firmness employing a Cutometer.
2.0 ELASTICITY AND VISCOELASTICITY CUTOMETER RESULTS
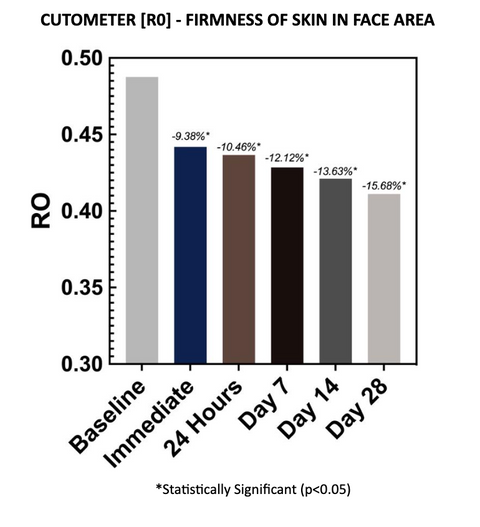
2.1 – RO READINGS
Cutometer readings (R0) show that the test material reduced final distention at each evaluation time point, with an average reduction of 15.68% after 28 days of twice-daily use. The decreases are considered statistically significant at all time points. A decrease in the R0 parameter is indicative of improved skin firmness, thickness, and tightness.
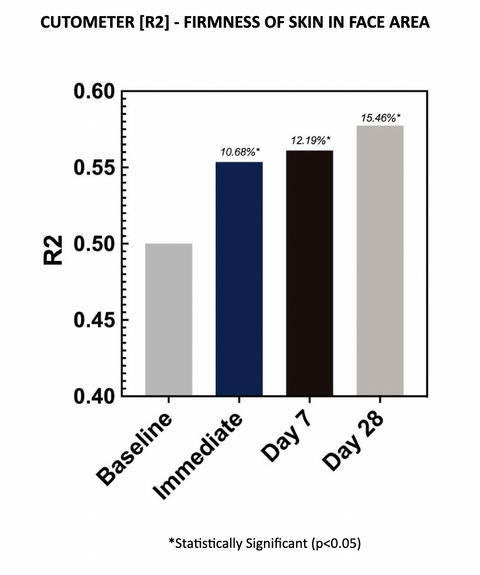
2.2 – R2 READINGS
Cutometer readings (R2) show the test material improved gross elasticity at each evaluation time point, with an average improvement of 15.46% after 28 days of twice-daily use. Increases in R2 parameters are indicative of improved skin elasticity. The increases are statistically significant at all time points.
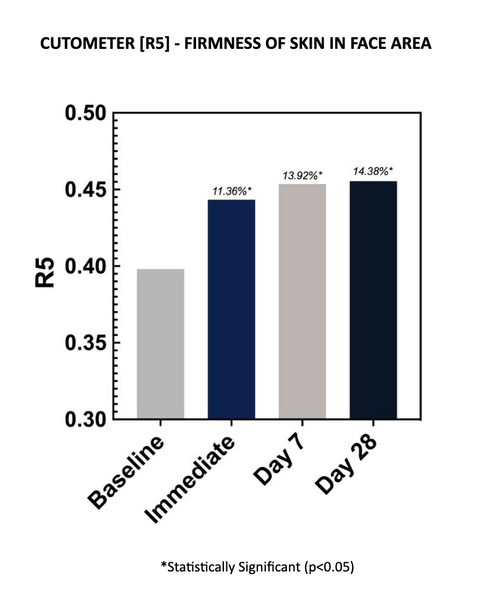
2.3 – R5 READINGS
Cutometer readings (R5) demonstrated improved net elasticity at each time point, with an average improvement of 14.38% after 28 days of twice-daily use. Increases in R5 parameters are indicative of improved skin elasticity. The increases are statistically significant at all time points.
2.4 – R7 READINGS
Cutometer readings (R7) demonstrated that the test material improved biological elasticity at each evaluation time point, with an average improvement of 12.30% after 7 days of twice-daily use. Increases in R7 parameters are indicative of improved skin elasticity. The increases are statistically significant at all time points.
3.0. TEST MATERIAL EVALUATION PRE-REQUISITE:
Prior to induction of a human test panel, toxicology, microbiology, or in-vitro performance spectra may be required to assess the feasibility of commencement as dictated by an Institutional Review Board (IRB).
3.1.1.
Sponsor purports that prior to sample submission, test materials were approved by the Sponsor’s Legal and Regulatory departments for inclusion in this testing program, and that the following tests were conducted with no adverse results and the test data are on file at their premises and have not been made available to personnel:
- USP or CTFA Preservative Efficacy Test or equivalent
- 90 Day Accelerated Stability and Container Compatibility Study
- Fifty (50) person Repeat Insult Patch Test (RIPT) or equivalent
4.0 INSTITUTIONAL REVIEW BOARD [IORG0011153] [IRB00013226]:
Reference: CFR Title 21 Part 56, Subparts A, B, C, and D. The IRB consists of 5 or more individuals, chosen for technical expertise and from the local community for lay interaction. The list of IRB members is kept on file and is available for inspection during regular hours of operation.
5.0 PANEL SELECTION:
5.1. KEY STANDARDS FOR INCLUSION IN THE STUDY
a. Females between 35 and 70 years old of varying ethnicities (skin types: all, I through VI).
b. Individuals, who read, understood, and signed an informed consent document as required by CFR Title 21, Part 50, Subpart B regulations.
c. Individuals who have abstained from using any anti-aging, sun-protecting and moisturizing products, including lotions, creams, and gels during the 7-day washout period and the entire duration of the study.
d. Individuals who agreed to use only the assigned test product during the test period.
5.2. KEY STANDARDS FOR EXCLUSION FROM THE STUDY:
a. Individuals who are under the care of a physician for conditions that would interfere with the test results, at the discretion of the Study Director.
b. Individuals currently taking medication that may mask or interfere with the test results, including the use of any anti-acne drugs, topical and oral retinoids, topical antibacterial agents, and any immunosuppressive drugs.
c. Individuals known to be participating in other clinical studies.
d. Individuals known to be employees of testing firms/laboratories or cosmetic/raw materials suppliers.
5.3. RECRUITMENT: Talk about your brand
Panel selection is accomplished by advertisements in local periodicals, community bulletin boards, phone solicitation, electronic media, or any combination thereof.
6.0 PANEL DEMOGRAPHICS:
Thirty-five (35) subjects were selected for this study:
Number of subjects enrolled ............................................................................... 35
Number of subjects completing study ................................................................. 34
Age Range ...................................................................................................... 45 - 69
Sex .................................................................... Female ..................................... 34
7.0 PROCEDURE:
A seven-day (7) washout phase was required prior to study commencement. During this phase, the daily wash procedure was standardized in all subjects. Participants were instructed to use only lab-issued soap. Additionally, as a condition of enrollment, only the subjects who abstained from using anti-aging, sun-protecting or moisturizing products throughout the entire study period including the washout phase were recruited for participation.
On the initial day of the study, upon arrival at the testing facility, subjects were required to familiarize themselves with, then sign an informed consent. Panelists were mandated to adhere to all the restrictions mentioned in the inclusion/exclusion sections. All participants were advised of the general nature and purpose of this study. The subjects were then acclimated to the ambient environment for a period not less than fifteen (15) minutes prior to baseline evaluation and biophysical measurements. The acclimation procedure was repeated for each subsequent evaluation time point
The study was conducted according to Sponsor’s requested design wherein all subjects received written and verbal instructions regarding product use and study restrictions. Subjects were required to use the test material as a part of their daily routine according to the following sponsor supplied instructions:
“Apply one (1) pre-primed drop of the serum morning and evening. To apply, twist the cap and watch the pre-primed pump in action. Remove the cap and press down to release the perfect amount. Rub into hands and gently apply onto the face.”
Each product was weighed and recorded on a Product Weight Log prior to study initiation and again upon study completion to establish a use determination.
SKIN ELASTICITY – CUTOMETER
(Courage+Khazaka, Model: SEM 575 SN: 32 98 1036)
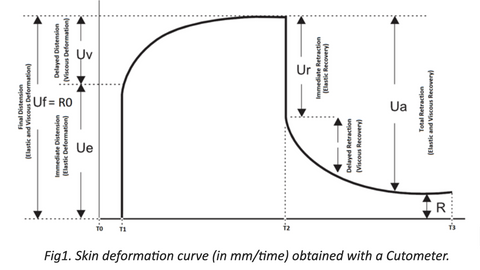
A Cutometer SEM 575 (Courage + Khazaka) is used to measure skin viscoelastic properties. The measuring principle of the Cutometer is based on a suction method, in which negative pressure, regulated between 20 and 500mbar, deforms the skin mechanically. Skin is drawn into a calibrated aperture (Suction Phase; T1-T2) and after a defined time released (Relaxation Phase; T2-T3), (refer to Fig.1). Inside the probe, penetration depth is determined by a non-contact optical measuring system, which consists of a light source and a light receptor as well as prisms facing each other. Light intensity varies due to penetration depth of the skin. Resistance of the skin to the negative pressure (firmness) and its ability to return into its original state (elasticity) are displayed as curves (refer to Fig.1) in real time during the measurement.
PHASE 1
SUCTION (when constant negative pressure is applied; T1 - T2)
During the suction phase, mechanical deformation is created. This results in skin’s immediate and delayed distention.
Immediate distention at T1 is described by Ue parameter and represents elastic deformation of the skin. Delayed distention, where skin “creeps” into the probe, takes place between T1 and T2 and is described by Uv parameter representing viscous deformation.
Elastic deformation together with viscous deformation is described by Uf (also called R0) parameter and represents maximum penetration of the skin into the probe.
Note: As skin elasticity increases, Uv decreases. Uv increases with age.
PHASE 2
RELAXATION (when negative pressure is cut off; T2 – T3)
During the relaxation phase, negative pressure is cut off. Complete relaxation is represented by the Ua parameter, which can be further divided into immediate elastic return (Ur) and delayed viscous return (Ua – Ur).
R2, R5, R7 and R0 parameters are evaluated during this study. R2, R5 and R7 address skin elasticity, and demonstrate negative correlation with age. R0 parameter addresses skin firmness and tightness. Additional R–parameters (R6, R8 and R9) may be included in this investigation at the discretion of study director.
R2
Gross elasticity (relative parameter), also called overall elasticity of the skin including the viscous deformation. R2 determines ability of the skin to return to its original state after deformation. The closer R2 is to 1 (100%) the more elastic the skin is.
R5
Net elasticity (without the viscous deformation) is a relative parameter between Elastic Recovery (Ur) and Elastic Deformation (Ue). R5 represents elastic recovery of the skin to return to its original position after deformation. The closer R5 is to 1 (100%) the more elastic the skin is.
R7
Biological elasticity (relative parameter). R7 determines ability of the skin to return to its original position after deformation. R7 is represented by the ratio of Elastic Recovery to Elastic and Viscous Deformation.
R0
Final Distension of the skin which represents passive behavior of the skin to applied force. It is an absolute parameter dependent on skin thickness. When R0 decreases, skin thickness, firmness, and tightness increase.
8.0 REFERENCES
gache, P.G., et al. “Mechanical properties and Young’s modulus of human skin in-vivo.” Arch. Dermatol. Res., 269, 221, 1980.
de Rigal, J. and Leveque, J.J. “In vivo measurement of the stratum corneum elasticity.” Bioeng. Skin, 1, 13, 1985.
Akhtar, N, et al. "Calendula extract: effects on mechanical parameters of human skin." Acta Pol Pharm 68.5 (2011): 693-701.